

Description
During a fireside chat with analysts from Brookline Capital Markets, Radiopharm Theranostics’ (ASX:RAD) CEO Riccardo Canevari provided clinical and corporate updates highlighting the company’s achievements while waiting for the completion of its listing on the NASDAQ expected at the end of 2024.
Canevari provided some updates about the joint venture with MD Anderson Cancer Center and the progress with the B7-H3 targeting radio-antibody (BetaBart). BetaBart is the first targeted radiopharmaceutical against the 4Ig subtype of B7-H3, the most common subtype expressed in human tumors.
“Management is initially targeting small cell lung cancer but sees additional opportunities in colon, renal, lung, cervical, prostate and glioma cancers,” Canevari said. The joint venture is expecting to undertake its first-in-human Phase 1/2 therapeutic trial by mid-2025.
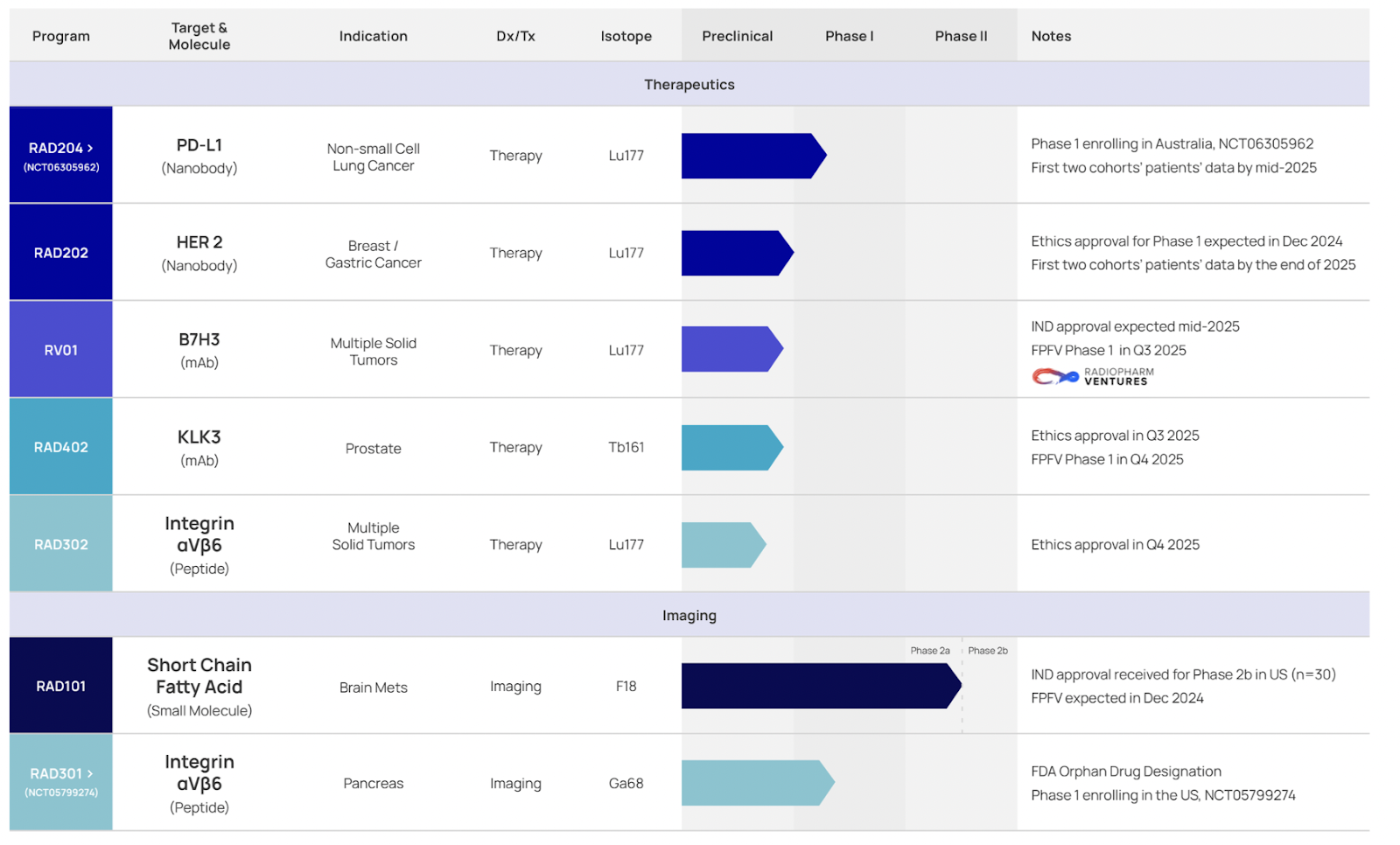
Canevari also outlined the progress of RAD’s focus programs including RAD101 for imaging brain metastases; RAD204 in NSCLC; RAD301 imaging in pancreatic cancer; and RAD202 in breast/gastric:
- RAD 101, an F18 radiolabeled Pivalate for brain metastases, received FDA IND approval for a Phase 2b trial aiming to enroll 30 patients, with the first dose expected in Q4 2024 and results anticipated mid-2025.
- RAD 204, a Lu-177-labeled nanobody targeting PD-L1 in NSCLC, recently dosed its first patient in a Phase 1 dose-escalating trial in Australia.
- RAD 301, a 68Ga-labeled αvβ6-integrin for imaging Pancreatic Ductal Adenocarcinoma, also recently dosed its first patient in a Phase 1 trial at Montefiore Medical Center, building on positive compassionate use data from 99 patients.
- RAD 402, targeting advanced prostate cancer with Tb-161, which has shown higher tumor uptake compared to Lu-177.
RAD initiated the process to obtain asecondary listing on the Nasdaq Capital Market in 2023, which the company expects to be completed by the end of 2024.
Highlights of the report
- Clinical Updates — RAD has advanced several clinical programs which include RAD 101 with the first dose expected in Q4 2024.
- Collaborations with Leading Industry Players — RAD has increased its ownership in Radiopharm Ventures to 75 percent from 51 percent.
- NASDAQ Listing Expected YE 2024 — RAD is progressing toward a secondary listing on the NASDAQ Capital Market by the end of 2024.
- Cash Runway Into June 2026 — In June 2024, RAD announced a capital raise of AU$70 million (USD $47million)
For the full analyst report, click here.
This content is intended only for persons who reside or access the website in jurisdictions with securities and other applicable laws which permit the distribution and consumption of this content and whose local law recognizes the scope and effect of this Disclaimer, its limitation of liability, and the legal effect of its exclusive jurisdiction and governing law provisions [link to Governing Law section of the Disclaimer page].
Any investment information contained on this website, including third party research reports, are provided strictly for informational purposes, are general in nature and not tailored for the specific needs of any person, and are not a solicitation or recommendation to purchase or sell a security or intended to provide investment advice. Readers are cautioned to seek the advice of a registered investment advisor regarding the appropriateness of investing in any securities or investment strategies mentioned on this website.

